Publications
Display by Type
Display by Topic
On the Detection of Carbon Fibre Storage Contamination and its Effect on the Fibre–matrix Interface
Scientific Reports 2018, 8, 16446.
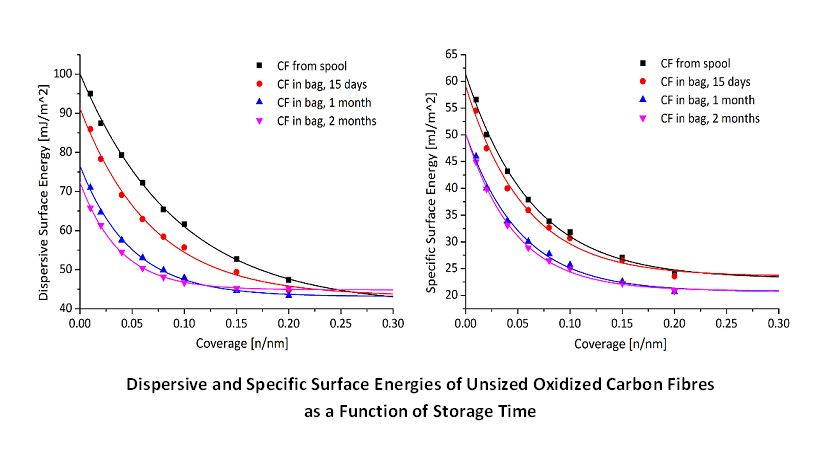
Abstract: Contamination caused by inappropriate carbon fibre (CF) storage may have an impact on their end use in reinforced composite materials. Due to the chemical complexity of CFs it is not easy to detect potential contaminants, especially at the early stage during manufacturing and handling. In this paper, X-ray Photoelectron Spectroscopy (XPS), Fourier Transform Infrared (FTIR) spectroscopy and Surface Energy Analysis (IGC-SEA) were used to assess the surfaces of CFs stored in polyolefin zip-lock bags for possible contamination. Only after over 2 months in-bag storage, was XPS capable of detecting a minor increase in nitrogen on the CF surface while FTIR revealed the presence of fatty acid amides and fatty acids, both associated with the storage media. However neither of these techniques were sensitive enough to show significant evolution of the amount of contamination as a function of storage time. In contrast, IGC-SEA distinguished surface energy differences between CFs before and after storage. These differences were found to change as a function of storage time, which were attributed to increases in contamination amounts. Single fibre fragmentation tests indicated that the surface contamination had potential to disrupt the fibre-matrix interface. These findings provide a new method for assessing the surface contamination of CFs with potential application to other materials.
Full Text
Confirmation of Bioinformatics Predictions of the Structural Domains in Honeybee Silk
Polymers 2018, 10, 776.
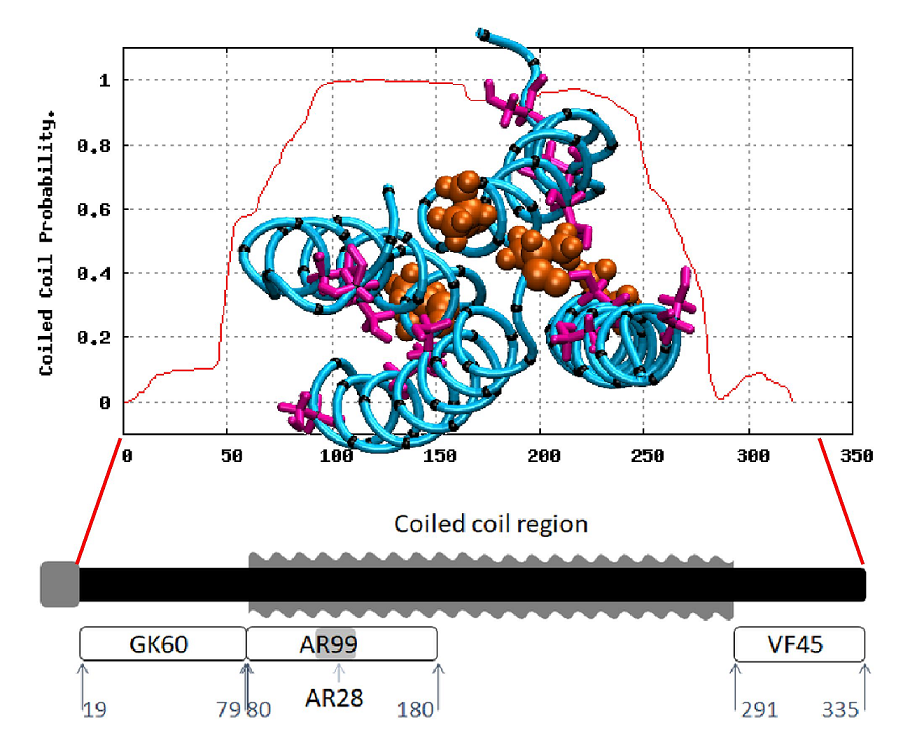
Abstract: Honeybee larvae produce a silk made up of proteins in predominantly a coiled coil molecular structure. These proteins can be produced in recombinant systems, making them desirable templates for the design of advanced materials. However, the atomic level structure of these proteins is proving difficult to determine: firstly, because coiled coils are difficult to crystalize; and secondly, fibrous proteins crystalize as fibres rather than as discrete protein units. In this study, we synthesised peptides from the central structural domain, as well as the N- and C-terminal domains, of the honeybee silk. We used circular dichroism spectroscopy, infrared spectroscopy, and molecular dynamics to investigate the folding behaviour of the central domain peptides. We found that they folded as predicted by bioinformatics analysis, giving the protein engineer confidence in bioinformatics predictions to guide the design of new functionality into these protein templates. These results, along with the infrared structural analysis of the N- and C-terminal domain peptides and the comparison of peptide film properties with those of the full-length AmelF3 protein, provided significant insight into the structural elements required for honeybee silk protein to form into stable materials.
Keywords: coiled coil; protein secondary structure; bioinformatics protein folding prediction; protein design; protein engineering; protein materials; infrared spectroscopy; circular dichroism spectroscopy; molecular dynamics; cast film solubility
Full Text